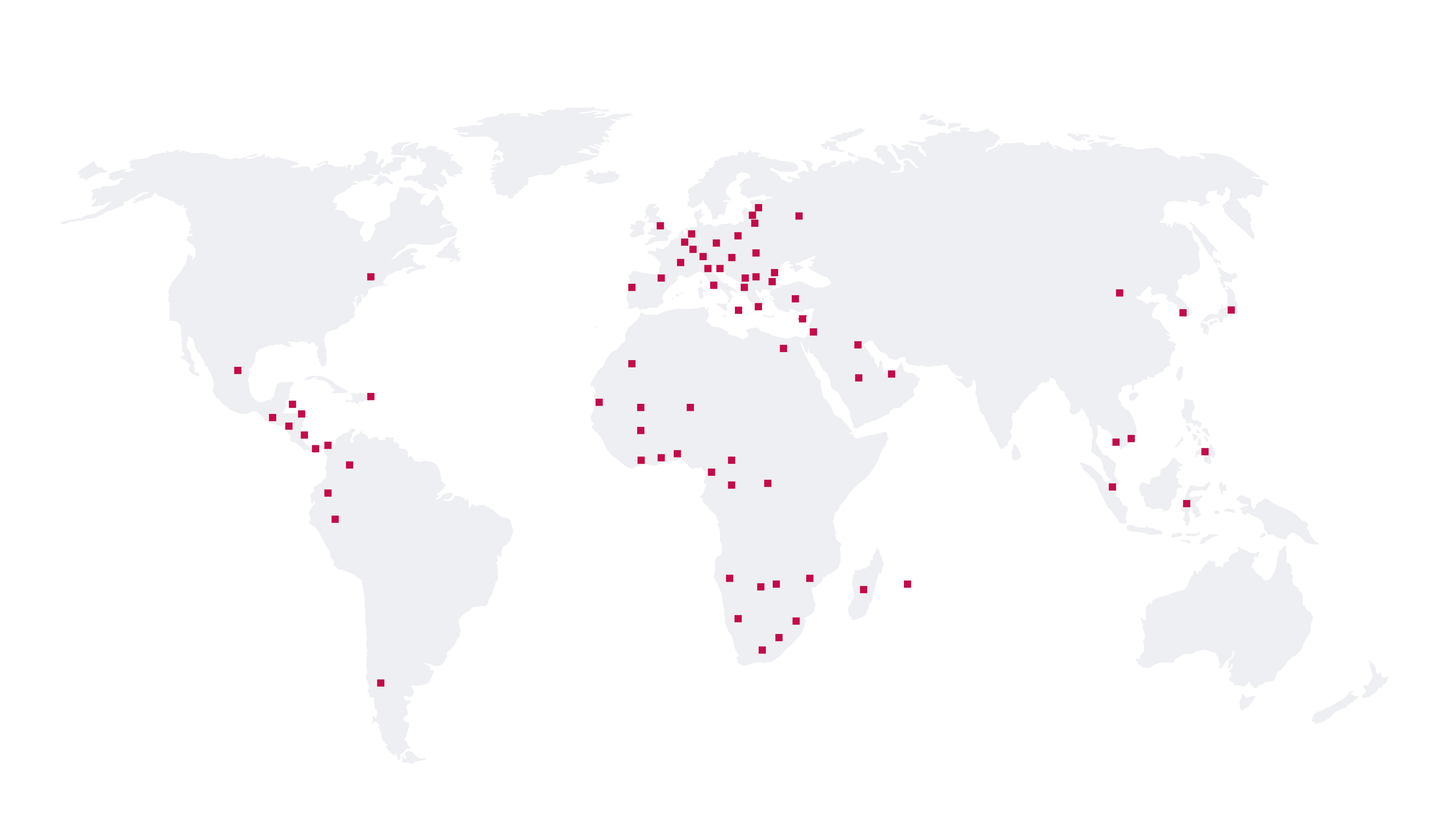
Together
for Health
Our Portfolio
Our most mature commercial portfolio consists of 3 main molecules: rupatadine, a second-generation antihistamine; flutrimazole, a topical antifungal; and triflusal, a platelet aggregation inhibitor.
RUPATADINE
Rupatadine is a second generation antihistamine indicated for the symptomatic treatment of allergic rhinitis and urticaria.
Rupatadine
TRIFLUSAL
Triflusal is a platelet aggregation inhibitor indicated in:
Secondary prophylaxis, after a first coronary or cerebrovascular ischaemic event, of:
- Myocardial infarction
- Stable or unstable angina
- Transient or permanent non-hemorrhagic stroke
Reduction of graft occlusion after coronary by-pass surgery
FLUTRIMAZOLE
Flutrimazole is an antifungal indicated for the topical treatment of superficial mycoses of the skin, such as:
- Ringworm caused by Trichophyton, Microsporum and Epidermophyton floccosum
- Cutaneous candidiasis mainly caused by yeasts of the genus Candida
- Pityriasis versicolor caused by Malassezia furfur, also known as Pityrosporum ovale.
Our strategic
therapeutic areas
Respiratory, Dermatology (including conditions with an inflammatory and allergic component) and Women's Health.
We have several new chemical entities in early stages of development, from preclinical to phase II clinical phase, all of them available for licensing.
All our new chemical entities are manufactured in our production facilities, from R&D to commercialisation.
| Preclinical | Phase I | Phase IIa | Phase IIb | Phase III | Registration | Launch | |||
|---|---|---|---|---|---|---|---|---|---|
|
Albaconazole
Vulvovaginal candidiasis
|
In progress |
||||||||
|
UR-63325
Histamine 4 Receptor Antagonist
|
|||||||||
|
UR-65318
Histamine 4 Receptor Antagonist
|
|||||||||
Generic products
Our generic business consists of two business units: APIs (Active Pharmaceutical Ingredients) and FDFs (Finished Dose Forms).
Product currently under patent protection could be available only for R&D purposes if this is allowed by local laws. Samples for R&D use in the United States are available as permitted under 35 USC § 271 (e)(1).
Products covered by valid patents in any country are not offered or supplied to these countries. However, the final responsibility lies exclusively on the buyer.
APIs Business
The APIs business is based on the development of active pharmaceutical ingredients, and their worldwide commercialisation through other companies.
All our APIs are manufactured in our chemical plant, URQUIMA, in compliance with the highest standards of good manufacturing practice (GMPs) and EHS (Environmental, Health and Safety).

Generic APIs Portfolio
Azelastine
Bilastine (**)
Roflumilast
Cilostazol
Glimepiride
Glipizide
Ivabradine (**)
Desvenlafaxine (**)
Zonisamide
Apremilast (*) (**)
Pirvinium pamoate (**)
Silodosin (**)
TIP, CEP, EU, US, KR, JP, CN and other DMF files available depending on the product
(*) under development
(**) Project with own Patent

Finished Product Business (FDFs)
The FDF Business is focused on the development of generic dossiers of oral pharmaceutical products (tablets, film-coated tablets and capsules). The dossiers are elaborated under the European Guidelines.
Generic versions of reference branded products provide a safe, effective, and cost-efficient alternative.
Main therapeutic areas
Central Nervous System, Cardiology, Respiratory and Urology.
Our value
Our extensive track record and chemical expertise in the development of new polymorphs, salts, cocrystals and value-added alternative formulations represent our competitive advantage, facilitating an early entry to the market and helping patients to secure faster access to affordable and quality healthcare.
Finished product portfolio
|
Aripiprazole Schizophrenia |
Levetiracetam Epilepsy |
Rasagiline Parkinson |
|
Desvenlafaxine XR Depression |
Memantine Alzheimer's |
Zonisamide (**) Epilepsy |
|
Bilastine (**) Allergic rhinitis |
Apremilast (*)(**) Psoriatic arthritis |
Roflumilast COPD |
|
Anagrelide Thrombocytosis |
Ezetimibe Hypercholesterolemia |
Rivaroxaban Deep vein thrombosis |
|
Cilostazol Intermittent Claudication |
Irbesartan Essential hypertension |
Ivabradine Angina pectoris |
|
|
|
|
Silodosin Prostate hyperplasia |
|
|
(*) Under development.
(**) Project with own patent,
Our offer
We are experts in the manufacture of oral solids (tablets, film-coated tablets, capsules) and semi-solids (ointments, creams and gels), which we develop and manufacture for other companies.
We also offer the development and manufacture of food supplements under GMP and ISO standards guaranteeing the highest quality.

Our Approach
We provide a first class service in terms of quality, reliability and cost through a collaborative model with a high degree of flexibility. We adapt to the demands of our clients by giving them personalised attention and adjusting our processes to their requirements.