Together
for Health
Our
facilities
Our customer focus and vertically integrated API and finished product manufacturing facilities enable us to offer fast turnaround times, operational excellence and cost optimisation.
URQUIMA
Our chemical plant
Urquima is the Noucor site where the active ingredients we develop are manufactured.
NOUCOR HEALTH
Pharmaceutical and food supplements plant
Noucor Health is the Noucor site where the head offices and the production plant for finished products, both medicines and food supplements, are located.
Urquima is the site of Noucor Group, S. L. where the active pharmaceutical ingredients (APIs) that we develop are manufactured. Our APIs are both generic and derived from our own development, for internal supply as well as for supplying other local and international pharmaceutical companies.
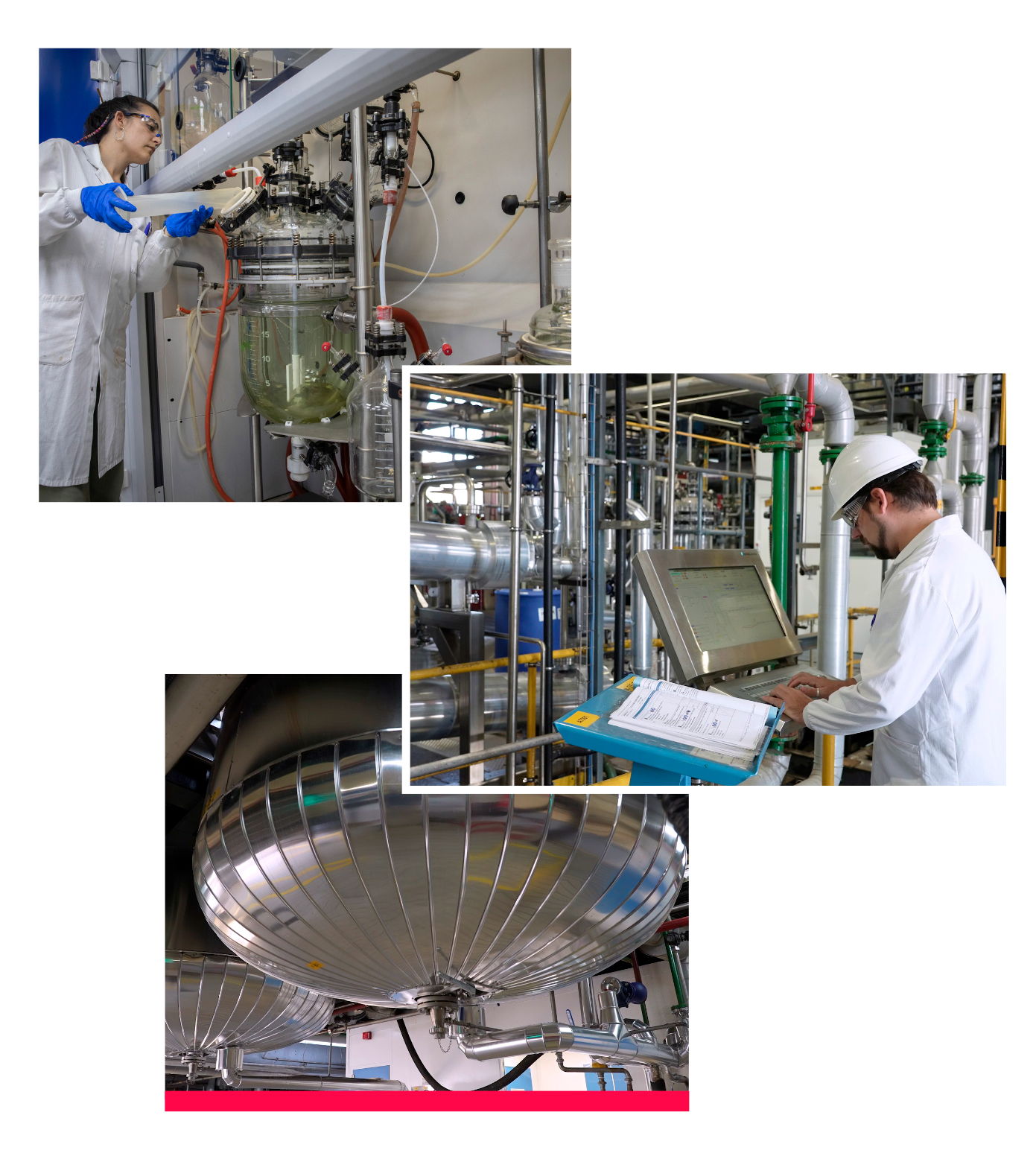
Urquima is a 12,800m2 multi-product manufacturing plant with facilities including: a kilolab, a pilot plant, a semi-industrial plant and an industrial plant, as well as R&D and quality control laboratories.
URQUIMA in figures:
- Production capacity of 56 tonnes/year.
- Maximum reaction volume of 130m3.
- Pilot plant equipped with multi-purpose reactors, clean rooms and chemical flow hydrogenator.
- Laboratories equipped with the main analytical technologies such as HPLC, UPLC, Gas Chromatography, Laser Diffraction for particle size determination, among others, and managed by the most modern and flexible LIMS on the market.
![]()




tonnes/year

Certifications
The manufacturing plant and its quality system complies with GMP, environmental and health and safety standards, ISO 14001 and ISO 45001, and is certified by the world's leading authorities including the US FDA, Japan's PMDA, the AEMPS and the Generalitat de Catalunya.

International
Our expert and proactive approach and ability to develop and manufacture non-infringing processes gives our customers a commercial competitive advantage allowing them to enjoy market exclusivity.


Noucor Health is the site of Noucor Group, S. L., where the head offices, a pilot plant and the production plant for the development and production of pharmaceutical products and food supplements are located.
Noucor has a space of 63,000 m2 in which the vertically integrated plants, equipped with the latest technology, occupy 20,000 m2. 370 employees work on this site every day.


NOUCOR HEALTH plant in figures:
The finished product pharmaceutical plant has the capacity to manufacture a variety of solid (such as tablets, coated tablets, lozenges, capsules) and semi-solid (creams, ointments, etc.) dosage forms.
Pharmaceutical production






Certifications
The manufacturing plant and its quality system complies with GMP, ISO 13485 and ISO 22716 and environmental and health and safety standards, ISO14001 and ISO 45001, and is certified by the world's leading authorities, including the Korean FDA, Japan's PMDA, the AEMPS and the Generalitat de Catalunya.
Food supplements






Facilities
The facilities also accommodate all areas necessary to achieve a turnover that already exceeds 100 million Euros. From preclinical and clinical development, patents, regulatory, chemical, pharmaceutical, analytical and quality control laboratories, quality assurance, EHS & sustainability, legal, finance, corporate services, commercial to people & communication and general management.